Que recherchez-vous ?
Un contenu sur ce site
Une personne sur l'annuaire
Une formation sur le catalogue
Un contenu sur ce site
Une personne sur l'annuaire
Une formation sur le catalogue
Study of Solid / Liquid Interface: Influence of the electrical double layer
This work, performed within the framework of the INTERACTIVE LABEX, and in partnership between the Department D1 Physics and Mechanics of Materials and D2 Fluid, Thermal and Combustion of the Pprime Institute, concerns the study of solid / liquid interface and particularly the behavior of the electrical double layer (EDL).
To this purpose, sophisticated methods of modifications of the surface morphology will be tested, as isotropic and anisotropic modifications of the roughness at different scales (nm, µm) by polishing process, ion beam sputtering, deposition of organized nanostructures (islands, ripples,..), Focuse Ion Beam (FIB) or other technics of surface structuration (e.g. lithography). The influence of the chemical modifications of the surface (by oxidation, nitriding, hydrogenation, metal implantation, deposition of noble metals…) as well as the influence of the crystallographic orientation will also be studied.
Different types of techniques are employed for the characterization of surface. XPS and EDS-WDS are used for surface chemical characterization while morphological characterization is carried out by SEM, WLI and AFM. Electrochemical Impedance Spectroscopy (EIS) and Cyclic Voltammetry (CV) are the techniques dedicated to the identification of the characteristics of the electrical double layer, especially its capacitance. In parallel, other electrical techniques of characterizations of the EDL are locally developed such as spinning disk and flow electrification experiments.
The first experiences developed in this work have focused on the feasibility of the identification by impedance spectroscopy, of the impact of the nature and structure of various surfaces on the distribution of charges at the interface and especially the EDL.
In a second time, the preparation of a “reference” polycrystalline austenitic stainless steel 304L surface has been achieved by optimizing the polishing and cleaning process.
In addition, during these first months, specific electrochemical cell has been developed allowing the study of the EDL without outside influences (poisoning). The figure 1 shows the electrochemical cell.
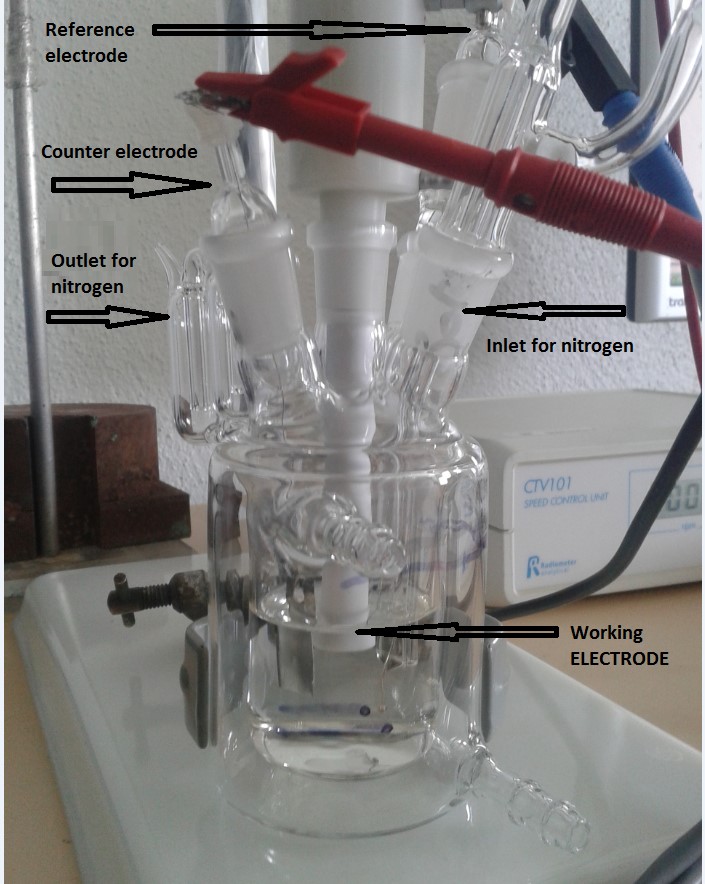
Today, the analytical capabilities of the CV and EIS techniques have been combined in this work to measure double layer capacitances of 304L electrode in a solvent NaCl concentrated to 0.01 M.
C = 2.62* 10-7 F
Rs = 251 ohm
Future work will focus on changing the interface, both solid side (morphological modifications of the material surface) and liquid side (electrolyte concentration, temperature …), to understand well the impact of the interface on the EDL. The aging of the material will be studied (XPS, WLI, AFM) and temporal monitoring of the electrolyte will be followed by different measuring techniques (chronoamperometry, conductivity measurement).